| |
| Research Projects |
| Lab Members |
| Publications |
| Gallery |
| Useful Links |
| Positions Available |
| Funding |
| Contact |
| Home |
| P23H Images Continued: |
The following results were described in our 2007 publication in J Neuroscience and our more recent 2009 publication in IOVS.
|
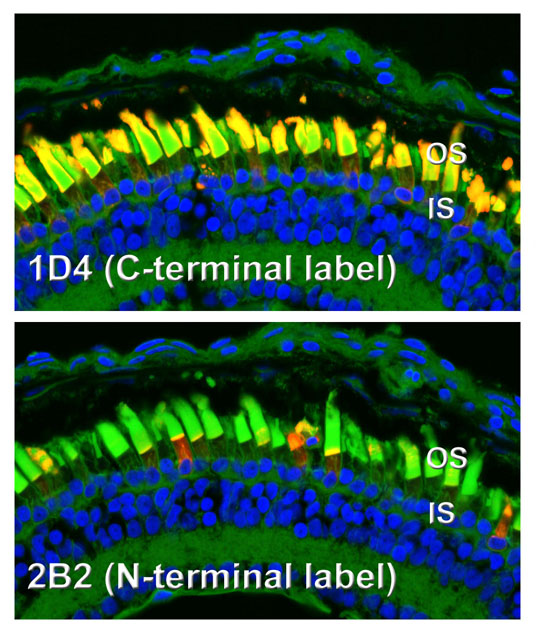
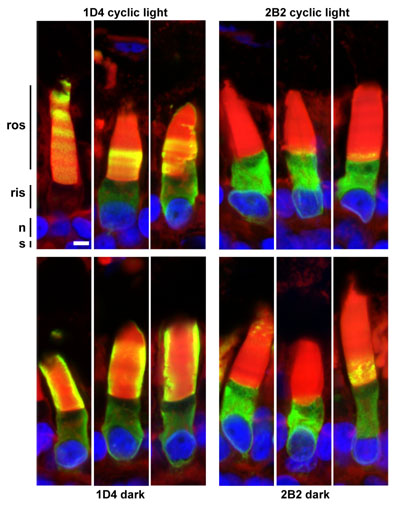
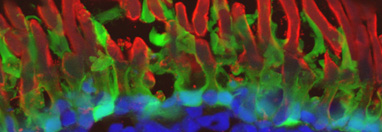
Transgenic photoreceptors expressing bovine P23H rhodopsin, demonstrating differential reactivity of N- and C-terminal antibody epitopes. While the C-terminus (1D4) is abundant in the outer segments, the N-terminal epitope (2B2) is largely absent from the outer segments, and relatively speaking, is much more abundant in inner segments. This is due to the fact that the N-terminus of P23H rhodopsin is cleaved on exit from the ER (confirmed in western blot to the right). In this image, the outer segments are counterstained with wheat germ agglutinin (green).
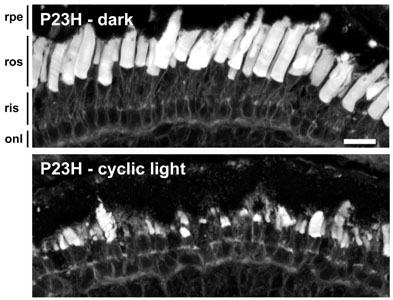
Complete rescue of retinal degeneration in animals expressing bovine P23H rhodopsin by dark rearing. The photoreceptors in the top panel appear quite normal, while most of the rods are missing in the lower panel. Retinal degeneration in animals expressing bovine rhodopsin is strongly influenced by light exposure, and chromophore binding is involved in the rescuing effects of dark rearing (see panel to the right). We are currently investigating whether this effect is also linked to the phenomenon of N-terminal cleavage.
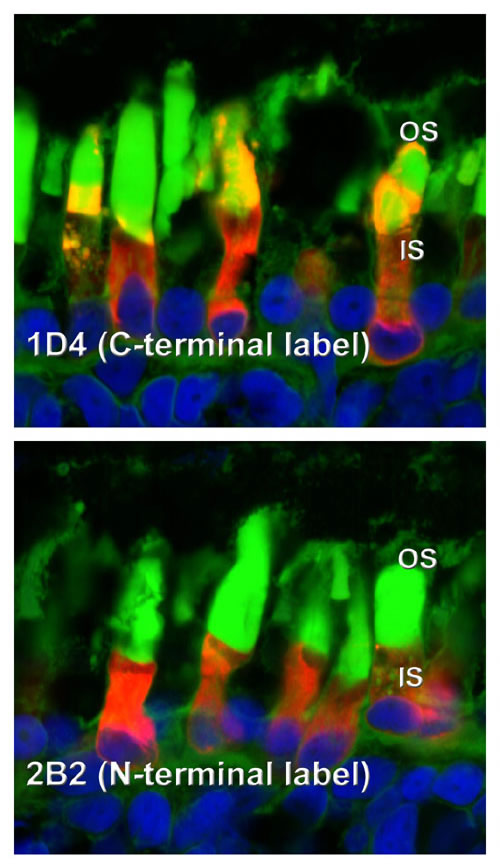
We obtained similar differential labeling with N- and C-terminal directed antibodies with human P23H rhodopsin expressed in X. laevis photoreceptors. The effect is not as dramatic, but clearly present.
We obtained a similar rescuing effect of dark rearing with human P23H rhodopsin. Again, the effect is not as dramatic, but clearly present. Dark rearing did not completely protect these animals from retinal degeneration.
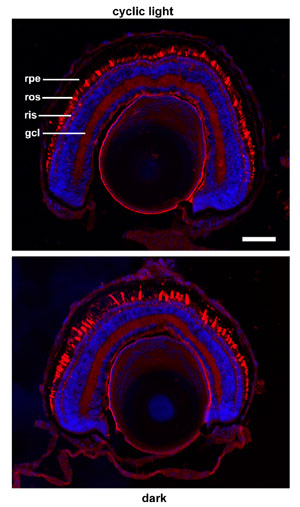
Greater quantities of cleaved P23H rhodopsin are transported to the rod outer segment under dark rearing conditions. Labeling with the C-terminal antibody 1D4 (left panels) shows more consistent and higher levels of transport of P23H rhodopsin to rod outer segments in the dark (bottom) than in cyclic light (top). Banding in the top panel also suggests a relationship between this transport and the light/dark cycle. However, virtually all of the rhodopsin transported to the outer segments is cleaved at the N-terminus, and cannot be labeled with the N-terminal antibody 2B2 (right panels). In total, our results suggest a synergy between chromophore binding and proteolytic cleavage that allows transport of P23H rhodopsin to the outer segment in the absence of light exposure.
|
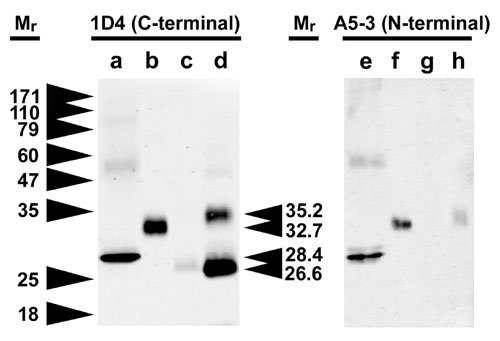
Western blot analysis of transgenic X. laevis eyes expressing bovine P23H rhodopsin confirming N-terminal cleavage. Samples are a) deglycosylated bovine rhodopsin or eyes expressing b) normal bovine rhodopsin c) low level expression of bovine P23H or d) high level expression of bovine P23H. In the second panel, the samples are repeated and probed with an N-terminal specific antibody. The P23H rhodopsin is clearly truncated relative to WT bovine rhodopsin, and no longer reacts with the A5-3 antibody. The difference is not simply due to lack of glycosylation.
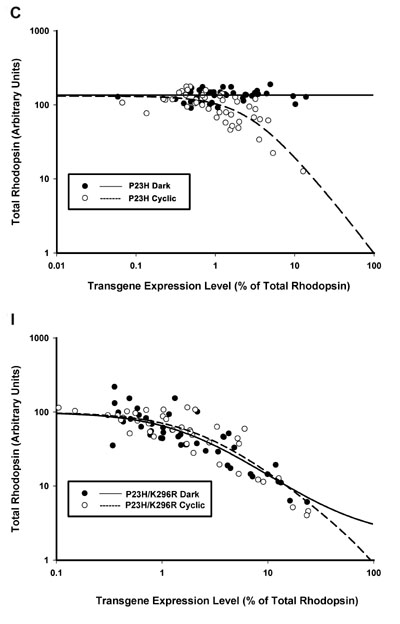
Demonstration of the involvement of rhodopsin chromophore in mediating the effects of light and dark on retinal degeneration. In the top panel, a strong effect of dark rearing is observed in animals expressing P23H rhodopsin. Dark reared animals retain high levels of total rhodopsin (i.e. healthy photoreceptors) even when expressing high levels of P23H transgene. However, when chromophore binding is disrupted using a second mutation (K296R), this effect is eliminated. Promoting retinal degenerative is a property intrinsic to the P23H protein and does not require light exposure. However, chromophore binding is necessary in order to provide the rescuing effects of dark rearing. It is unclear whether chromophore binding and N-terminal cleavage co-operate in this process.
|
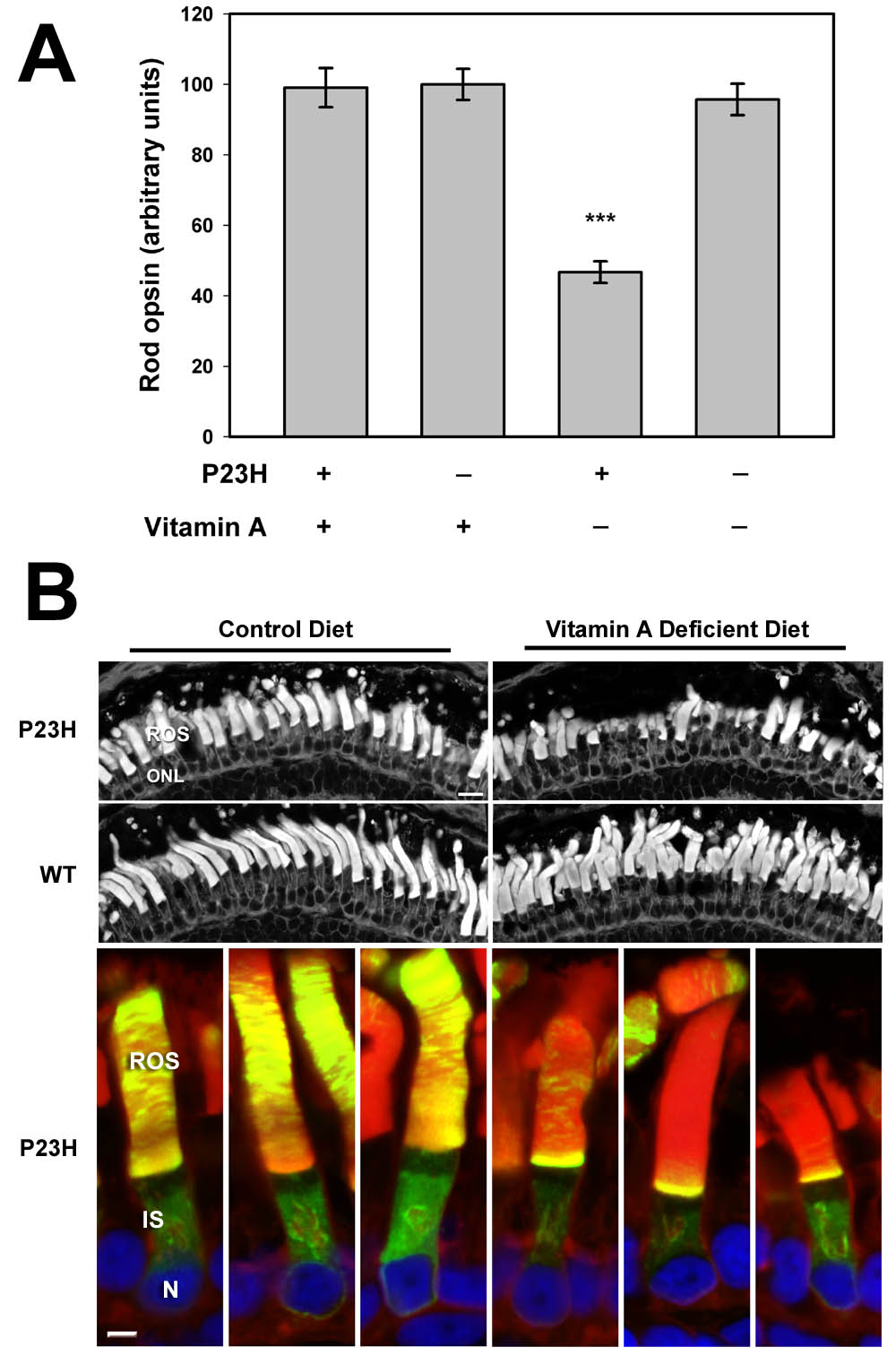
Dependence of retinal degeneration on vitamin A. Normal and P23H animals were reared in the dark on either a control diet or a diet deficient in Vitamin A. Only the P23H animals on the vitamin A deficient diet showed decreased total opsin levels (A) and retinal degeneration by histology (B). The vitamin A deficient diet also greatly reduced the delivery of P23H rhodopsin (green) to the rod outer segments (B, lower panesl).